Cryolock® Vitrification Device for Oocyte and Embryo Cryopreservation
CryoLock® vitrification devices are premium quality, versatile, simple and efficient vitrification devices intended for use during IVF procedures for the holding, cryopreservation and storage of oocytes or embryos in liquid nitrogen. CryoLock® is FDA 510(k) Cleared, CE Mark Approved and Health Canada Approved.
(Looking for something slimmer? Check out the new S-Cryolock!)
Design:
CryoLock® is a square stick-shape device, with four flat surfaces. Both the cap and body possess the same coefficient of expansion ensuring an equally secure coupling at room temperature as well as low cryogenic temperatures.
Body and cap have gaps on their extremes, which allow easy grip with forceps during manipulation.
Material:
CryoLock® components, the body and cap, are made of polystyrene medical grade plastic, which facilitates even temperature conduction from side to side of the device.
CryoLock® and its secure cap were designed in order to ensure maximum safety of the specimens during storage. CryoLocks are available in sterile and sealed vials of 5 Cryolock units, ready to use in human IVF and animal research laboratories.
CryoLock® features are:
- Manufactured in a single piece and plastic injection between 180ºC and 260ºC avoiding contamination and presence of impurities.
- Slots at the ends of the CryoLock® body and cap for better manipulation avoid the risk of loose caps during their storage.
- Exclusive cap design for a perfect fit at room and freezing temperatures.
- Ample surface for labeling facilitates sample identification.
- Available on vials of five CryoLock® units duly sterilized by gamma radiation.
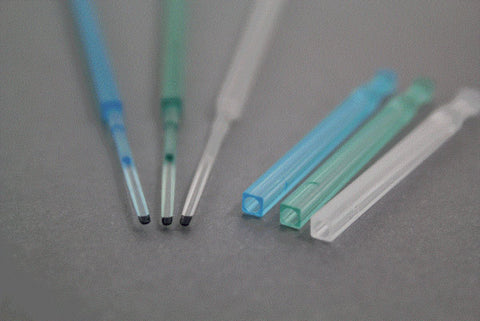 CryoLock® Tip
CryoLock® Tip
Its concave tip, where the oocytes or embryos are placed, provides protection to the sample against contact with other surfaces, avoiding loss or damage of specimens.
CryoLock® Closure System
Its unique design allows an hermetic seal which is created by a tapered surface where the Cryolock body and cap fit in a perfect seal creating a closure system able to keep the tip isolated from liquid nitrogen ( LN2) once the Cryolock is submerged into the LN2.

While Cryolock is under LN2 the cap shrinks over the sealing area due to the low temperatures. While specimens are stored LN2 cannot recirculate inside the cap towards the tip or vice versa.
When Cryolock is used as a Semi-Closed System, the ONLY time specimens are in direct contact with LN2 is during capping step under LN2. In this case is recommended to use individual batches of LN2 for patients in order to reduce the risk of cross-contamination during cryopreservation procedures.
When Cryolock is used as a Closed System specimens are never in direct contact with LN2 thus avoiding the risk of cross-contamination during cryopreservation procedures and storage conditions.
Cryolock use instructions [pdf]
Cryolock is FDA 510(k) Cleared, CE Mark Approved and Health Canada Approved.
Cryolock® is a Cryopreservation Storage device that is intended for use in vitrification procedures to contain and maintain human 1-cell stage embryos. In non-US countries: for Oocytes and/or Embryos.
Specifications:
| Mfr. No. CL-R-CT-B | Cryolock® Vitrification Device. 50 Cryolocks (10 packs of 5). Blue. |
| Mfr. No. CL-R-CT-Y | Cryolock® Vitrification Device. 50 Cryolocks (10 packs of 5). Yellow. |
| Mfr. No. CL-R-CT-C | Cryolock® Vitrification Device. 50 Cryolocks (10 packs of 5). Clear. |
| Mfr. No. CL-R-CT-O | Cryolock® Vitrification Device. 50 Cryolocks (10 packs of 5). Orange. |
| Mfr. No. CL-R-CT-G | Cryolock® Vitrification Device. 50 Cryolocks (10 packs of 5). Green. |









 CryoLock® Tip
CryoLock® Tip

